Advantiflex 4-Way Turn Mattress Covers
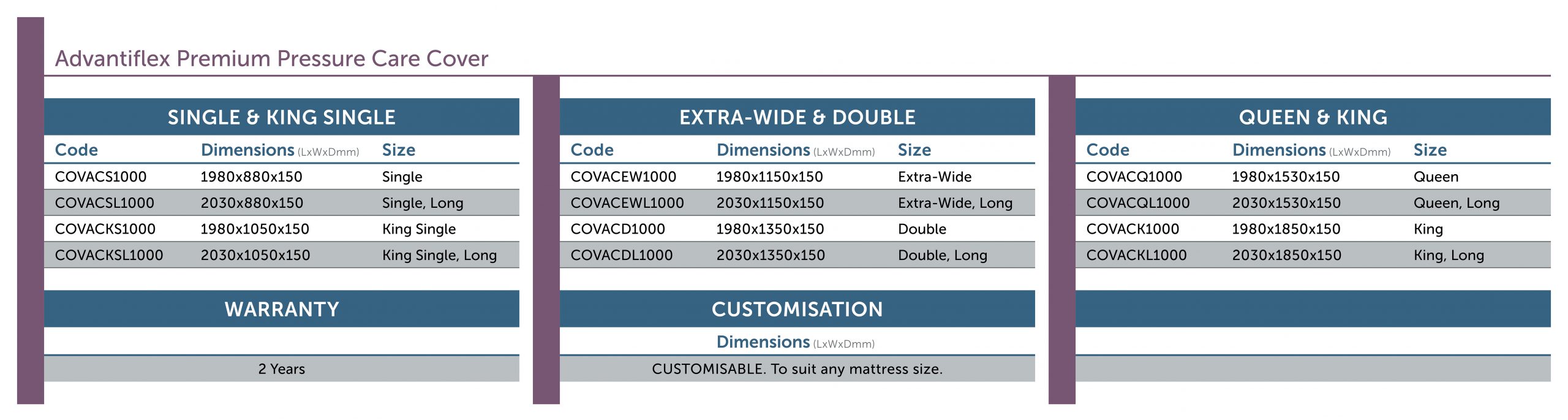
Advantiflex Premium Pressure Care Cover
The Advantiflex Pressure Care Cover is a tried and proven medical grade pressure care cover suited for use in all healthcare settings. The Fully Sealed design with all seams high frequency welded ensures the mattress is suitable for mental health and high risk of infection applications. Utilising raw materials unique to Forté Healthcare produced in Belgium, the Advantiflex range offers excellent value, compliance, infection control, performance, and patient care. Pressure injury prevention is delivered through bi-directional stretch qualities and moisture vapour permeability.
Features

ADVANTIFLEX PU1100 POLYURETHANE PRESSURE CARE MATERIAL
- High Performance, Single Sided Polyurethane
- 4-Way/Bi-Directional stretch for shear protection, immersion & envelopment
- Vapour permeable and breathable
- Resistant to patient body fluids
- Flexible and gentle to the skin
- Standard Colour: Blue

CLEANING AND CARE
- 1,000ppm disinfection chemical suitability
- Wipe clean
- Stain Resistant
- Anti-microbial protected

COMPLIANCE
- Fire retardant, meets BS 6807 ignition source 5 and BS 7175 ignition
- source 5 standards
- Anti-microbial protected
- All seams high frequency welded, no exposed sewn seams
- Meets and Exceeds Facility Requirement
- Materials manufactured in Belgium, to Forté specification

FULL VAPOUR PERMEABILITY
- Allows vapour to travel right through the cover at a controlled rate
- Reduces risk of internal infection entrapment and optimises core micro-climate

HIGH FREQUENCY SEALED AND WELDED SEAMS
- Electromagnetically fused seams protecting against risk of penetration of fluid
- Meets and exceeds infection control requirements
- Reinforced with additional heavy duty stitching at stress points
- 100% Sealed to prevent ingress of fluid or infection

CONSTRUCTION OPTION 1 – FULLY SEALED & ENCLOSED
- No-Zipper
- All seams welded and 100% waterproof to prevent ingress of fluid and infection (high frequency sealed and welded seams)
- Ultimate core durability and protection,
- Used for mental health – protects foam core against willful damage
- Used in high risk infection control applications
- Usually manufactured with an integrated pressure relief system

CONSTRUCTION OPTION 2 – INTEGRATED ZIPPER W/ WATERFALL PROTECTION FLAP
- 180° / Two-Sided Zipper
- Allows easy cover removal
- Robust and durable
- High frequency welded attachment for optimal protection against fluid ingress
- Waterfall Fluid Penetration Flap protects additional protection against fluid ingress through zipper
- TGA Compliant Product Labelling

CONSTRUCTION OPTION 3 – CUSTOM ZIPPER
- up to 360° / Four-Sided Zipper
- Other custom zippers available – contact Forté and provide them with the details of what you require. i.e. water resistant, invisible.
- Image for illustration purposes only
About Advantiflex
Pressure Care Covers
4-Way Stretch

4-Way Turn

Soft Feel

Vapour Permeable
<h3>INFECTION CONTROL</h3>
Effective infection prevention and control are central to providing high quality healthcare for patients and a safe working environment for those who work in healthcare settings. The Australian Guidelines for the Prevention and Control of Infection in Healthcare (AGPIH) provide evidence-based recommendations that outline the critical aspects of infection prevention and control, focusing on core principles and priority areas for action.
The AGPIH describe the best way to prevent and reduce infections occurring in healthcare settings including resistant infections. The AGPIH include how to manage common infectious agents, for example, gastrointestinal viruses and evolving infectious agents, for example, influenza or multi-drug resistant organisms.
Any infectious agents introduced into the body can establish infection. In all healthcare settings, reusable medical devices, such as mattresses, should be handled in a manner that will prevent patients, healthcare workers and environmental contact with potentially infectious material. Post COVID-19 pandemic, Cleaning and infection control have become a bigger concern than ever before.
Appropriate reprocessing or precautions must be implemented for mattresses and other reusable equipment, to prevent patient-to-patient transmission of infectious agents.
Principles of reprocessing reusable medical devices include:
<ul>
<li>Only Therapeutic Goods Administration (TGA)-included reusable medical devices should be used; before purchase, healthcare facilities should ensure that manufacturer’s reprocessing instructions are provided and are able to be followed by the healthcare facility.</li>
<li>All reusable medical devices and patient-care equipment used in the clinical environment must be reprocessed according to their intended use and the manufacturer’s advice.</li>
</ul>
However, Other standard precautions must take place. Standard precautions are basic infection prevention and control strategies that apply to everyone, regardless of their perceived or confirmed infectious status. Strategies include hand hygiene, personal protective equipment, cleaning, and appropriate handling and disposal of sharps. These are the first-line approach to infection prevention and control in health service organisations and are routinely applied as an essential strategy for minimising the spread of infections. Standard precautions minimise the risk of transmission of infectious agents from one person or place to another, even in high-risk situations, and render and maintain objects and areas as free as possible from infectious agents
Specifically, in relation to the support surface, there should be no exposed materials that will absorb fluid. As such, every mattress cover in the facility environment must have high-frequency welded seams (as opposed to sewn) to meet infection control requirements and prevent seepage of fluid through open sewn seams which leave thousands of tiny holes in the seam. These electromagnetically fused seams protect against the risk of penetration fluid as they 100% seal off to prevent ingress of fluid or infection. To ensure extra strength, Forté reinforces welded seams with additional Intra-weld heavy duty stitching at stress points within the cover.
In addition, Forté mattress covers, and foam cores are inherently treated with anti-microbial agents to prevent the growth of mould and bacteria on soft surfaces. It is a recommendation that soft surfaces must be impregnated with antimicrobial (self-disinfecting) materials.
All Forté products are included and registered on the Australian Register of Therapeutic Goods (ARTG). Unless exempt, reusable medical devices must be ‘included’ in the ARTG before they may be supplied in Australia.
Forté mattresses can withstand stringent infection control procedures and have been designed to suit facility standards so you can have peace of mind that all areas of your facility are safe, clean environments for staff and patients.

UNIQUE FORTÉ FORMULATION
Forté has developed an exclusive range of fabrics that are manufactured in Belgium and converted by Forté into Pressure Care covers in our Armidale NSW manufacturing facility. Close collaboration with clinicians, years of experience in developing support surfaces for the medical application and partnerships with leading medical fabric producers, has allowed Forté to create these unique fabrics. Forté specified properties such as vapour permeability, stretch, and longevity create some of the highest performing covers available.
What is the benefit of using covers with fabrics specified by Forté? Guarantee that materials used are leading cover technology designed specifically to suit Australian standards and care environments.

RESISTANCE TO CLEANING AGENTS
Cleaning and infection control is now a bigger concern than ever before. However, it is important that soft surfaces, such as mattresses, are cleaned using the correct cleaning agents otherwise there is risk of damaging the equipment or spoiling it to the extent that it becomes an infection risk. For example, Usage of high concentrations of Phenol-based and Chlorine chemicals or abrasive cleaning agents can have a negative impact on the integrity of the mattress fabric coatings over time.
Forté Premiflex fabrics have been designed to have a high resistance to cleaning agents which means a broader range of strong cleaning chemicals can be used to clean the surface without compromising on the performance and longevity of the mattress.
Premiflex fabrics have been designed and tested to withstand cleaning agents with 15,000ppm of active chlorine. Forté stipulates a maximum chlorine concentration of 10,000ppm for all Premiflex fabrics. As such, facilities can have confidence that their covers will perform under strong and consistent cleaning regimes – and more chance of cover-material longevity when the wrong cleaning agent or method is used – a common scenario in any care environment.
Premiflex covers provide the highest available level of infection control for a polyurethane pressure care cover.
VAPOUR PERMEABILITY
According to the Prevention and Treatment of Pressure Injuries: Clinical Practice Guideline, The International Guideline 2019 – Trans-epidermal water lows (TEWL) and sweating may result in the accumulation of moisture between the individual and the support surface if the surface does not allow for transmission or evaporation of the moisture away from the interface. The combination of a foam mattress cover and the foam itself creates resistance to moisture transmission. Moisture accumulation will increase the friction between the individual and the surface. A mattress with a high vapour transmission rate (MVTR) potentially allows the moisture to transpire through the cover. A lower mattress cover MVTR protects the foam from moisture degradation. Selecting a cover based on MVTR becomes a compromise between managing skin microclimate and the individual’s TEWL. It is recommended that a high specification mattress should have a cover with an MVTR ≥ 300g/m2/24hrs (equivalent to normal TEWL).
On the Advantiflex Classic cover, The PU1100 Upper material is tested to 600g/m2/24hrs.


Manufacturing
Forté Healthcare is the only Australian manufacturer producing specific medical grade covers, foam products and air systems within one single factory. This breadth of technology ensures a perfect alliance of technology for covers, foams and air systems.
Within the clinical setting, this ensures a better result for immersion, envelopment, microclimate, comfort and other factors necessary for skin integrity. This culmination of technology benefits other medical applications served by Forté including mental health, WH&S for Caregivers.
Forté proudly manufactures all elements of the high frequency welded covers within the Armidale factory. This ensures excellent quality control, efficiency, ability to customize and perfect compatibility with other components of the support surface.

You’re Covered by Forté Healthcare’s ‘Successful Solution Guarantee’
At Forté Healthcare, we are so confident in the care and performance our support surfaces deliver, that we back our products and expertise with our ‘Successful Solution Guarantee’.
Here’s how it works:
Our team of specialists will help you navigate our product range to correctly match our support surfaces with your care environment and patient risk profile. This ensures you achieve the highest levels of patient care and pressure injury prevention.
If, for whatever reason the product we recommend isn’t helping you achieve the outcome you expected, we’ll work with you until it is. And in the unlikely event we still cannot provide the right solution.
We’ll refund you 100% of the charges to date.