Heavy Duty PVC2 Waterproof Covers
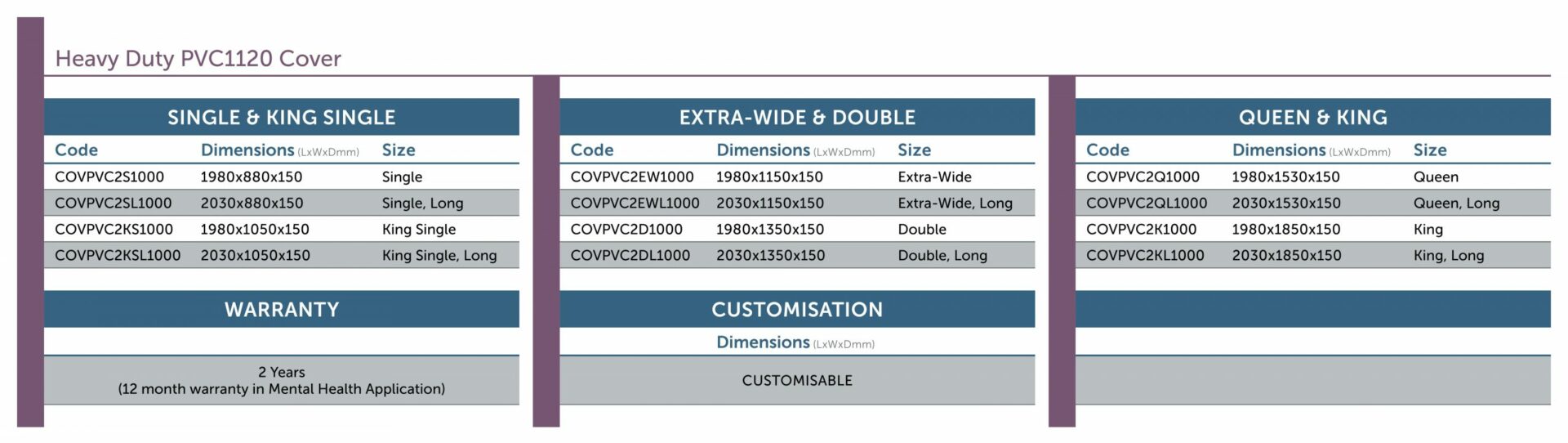
Heavy Duty PVC2 Cover
The Heavy-Duty PVC cover is manufactured with a 100% waterproof, durable PVC material. This high performing cover is designed for high demanding patient care scenarios from inpatient mental health to group home care. This cover is designed as a damage resistant cover and does not offer pressure care.
Features

EXTRA HEAVY DUTY PVC1120 MATERIAL
- 100% Waterproof
- Water Repellent
- Heavy Duty, Robust & Hardwearing
- Protects foam
- Superior resistance to cleaning agents
- Standard Colour: Grey

CLEANING AND CARE
- 10,000ppm disinfection chemical suitability
- Wipe clean
- Stain Resistant
- Anti-microbial protected

COMPLIANCE
- Fire retardant, meets BS 6807 ignition source 5 and BS 7175 ignition
- source 5 standards
- Anti-microbial protected
- All seams high frequency welded, no exposed sewn seams
- Meets and Exceeds Facility Requirement

HIGH FREQUENCY SEALED AND WELDED SEAMS
- Electromagnetically fused seams protecting against risk of penetration of fluid
- Meets and exceeds infection control requirements
- Reinforced with additional heavy duty stitching at stress points
- 100% Sealed to prevent ingress of fluid or infection

CONSTRUCTION OPTION 1 – FULLY SEALED & ENCLOSED
- No-Zipper
- All seams welded and 100% waterproof to prevent ingress of fluid and infection (high frequency sealed and welded seams)
- Ultimate core durability and protection,
- Used for mental health – protects foam core against willful damage
- Used in high risk infection control applications
- Usually manufactured with an integrated pressure relief system

CONSTRUCTION OPTION 2 – INTEGRATED ZIPPER W/ WATERFALL PROTECTION FLAP
- 180° / Two-Sided Zipper
- Allows easy cover removal
- Robust and durable
- High frequency welded attachment for optimal protection against fluid ingress
- Waterfall Fluid Penetration Flap protects additional protection against fluid ingress through zipper
- TGA Compliant Product Labelling

CONSTRUCTION OPTION 3 – CUSTOM ZIPPER
- up to 360° / Four-Sided Zipper
- Other custom zippers available – contact Forté and provide them with the details of what you require. i.e. water resistant, invisible.
- Image for illustration purposes only
About PVC2
Heavy Duty Cover

4-Way Rotational

High Durability
Resistance to Cleaning Agents

Waterproof
RESISTANCE TO CLEANING AGENTS
Cleaning and infection control is now a bigger concern than ever before. However, it is important that soft surfaces, such as mattresses, are cleaned using the correct cleaning agents otherwise there is risk of damaging the equipment or spoiling it to the extent that it becomes an infection risk. For example, Usage of high concentrations of Phenol-based and Chlorine chemicals or abrasive cleaning agents can have a negative impact on the integrity of the mattress fabric coatings over time.
Forté Premiflex fabrics have been designed to have a high resistance to cleaning agents which means a broader range of strong cleaning chemicals can be used to clean the surface without compromising on the performance and longevity of the mattress.
Premiflex fabrics have been designed and tested to withstand cleaning agents with 15,000ppm of active chlorine. Forté stipulates a maximum chlorine concentration of 10,000ppm for all Premiflex fabrics. As such, facilities can have confidence that their covers will perform under strong and consistent cleaning regimes – and more chance of cover-material longevity when the wrong cleaning agent or method is used – a common scenario in any care environment.
Premiflex covers provide the highest available level of infection control for a polyurethane pressure care cover.
INFECTION CONTROL
Effective infection prevention and control are central to providing high quality healthcare for patients and a safe working environment for those who work in healthcare settings. The Australian Guidelines for the Prevention and Control of Infection in Healthcare (AGPIH) provide evidence-based recommendations that outline the critical aspects of infection prevention and control, focusing on core principles and priority areas for action.
The AGPIH describe the best way to prevent and reduce infections occurring in healthcare settings including resistant infections. The AGPIH include how to manage common infectious agents, for example, gastrointestinal viruses and evolving infectious agents, for example, influenza or multi-drug resistant organisms.
Any infectious agents introduced into the body can establish infection. In all healthcare settings, reusable medical devices, such as mattresses, should be handled in a manner that will prevent patients, healthcare workers and environmental contact with potentially infectious material. Post COVID-19 pandemic, Cleaning and infection control have become a bigger concern than ever before.
Appropriate reprocessing or precautions must be implemented for mattresses and other reusable equipment, to prevent patient-to-patient transmission of infectious agents.
Principles of reprocessing reusable medical devices include:
<ul>
<li>Only Therapeutic Goods Administration (TGA)-included reusable medical devices should be used; before purchase, healthcare facilities should ensure that manufacturer’s reprocessing instructions are provided and are able to be followed by the healthcare facility.</li>
<li>All reusable medical devices and patient-care equipment used in the clinical environment must be reprocessed according to their intended use and the manufacturer’s advice.</li>
</ul>
However, Other standard precautions must take place. Standard precautions are basic infection prevention and control strategies that apply to everyone, regardless of their perceived or confirmed infectious status. Strategies include hand hygiene, personal protective equipment, cleaning, and appropriate handling and disposal of sharps. These are the first-line approach to infection prevention and control in health service organisations and are routinely applied as an essential strategy for minimising the spread of infections. Standard precautions minimise the risk of transmission of infectious agents from one person or place to another, even in high-risk situations, and render and maintain objects and areas as free as possible from infectious agents
Specifically, in relation to the support surface, there should be no exposed materials that will absorb fluid. As such, every mattress cover in the facility environment must have high-frequency welded seams (as opposed to sewn) to meet infection control requirements and prevent seepage of fluid through open sewn seams which leave thousands of tiny holes in the seam. These electromagnetically fused seams protect against the risk of penetration fluid as they 100% seal off to prevent ingress of fluid or infection. To ensure extra strength, Forté reinforces welded seams with additional Intra-weld heavy duty stitching at stress points within the cover.
In addition, Forté mattress covers, and foam cores are inherently treated with anti-microbial agents to prevent the growth of mould and bacteria on soft surfaces. It is a recommendation that soft surfaces must be impregnated with antimicrobial (self-disinfecting) materials.
All Forté products are included and registered on the Australian Register of Therapeutic Goods (ARTG). Unless exempt, reusable medical devices must be ‘included’ in the ARTG before they may be supplied in Australia.
Forté mattresses can withstand stringent infection control procedures and have been designed to suit facility standards so you can have peace of mind that all areas of your facility are safe, clean environments for staff and patients.

FIRE RETARDANT
The mental health care environment has patients with special needs that may differ from the acute care environment. Patients typically suffer mental illnesses which is a health condition that involves changes in emotion, thinking or behaviour (and/or a combination of these). Mental illnesses are associated with distress and/problems functioning in social, work or family activities. There can be a lot of risks involved with patients that have poor mental health – including self and property harm, for example, risk of fire.
As such, it is a standard for mental health medical equipment for all equipment to be anti-ligature and fire retardant.
For a mattress or support surface to truly meet mental health standards, All materials used must be fire retardant, including what is inside the cover.
What does Fire Retardant mean? A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion.
All foams used in Forté mental health mattresses meet BS5852 Source 5 Specification. Covers meet BS 6807 ignition source 5 and BS 7175 ignition source 5 standards.
What is BS5852 Source 5 Specification?
Test methods for assessing the ignitability of covers and fillings used in upholstered products such as mattresses, by smouldering and flaming ignition sources
BS5852 is a British Standard that describes methods for assessing the ignitability of single material combinations, e.g. cover and foam core of a mattress, when subjected either to a smouldering cigarette or to flaming ignition sources of thermal output ranging from that of a burning match to that approximating to the burning of four double sheets of full-size newspaper, as might be applied accidentally to any item of furniture.
Source five is one out of seven ignition sources of the BS5852;
| Source | Combustion type | Ignition source | Details | Energy inputs, Kw.h |
| 5 | Flaming | Crib | 17 g | 0.08 |
What is BS 7175 ignition source 5 Specification?
Methods of test for the ignitability of bedcovers and pillows by smouldering and flaming ignition sources. BS 7175 describes methods of test for the ignitability of bedcovers and pillows when subjected to smouldering and flaming types of ignition sources of differing severities.
Source five uses a wood crib 5. A crib is composed of wooden planks glued together. Lint is attached to the bottom. After adding propane-diol, the crib is placed on the test rig and ignited with a match. If no flaming or progressive smouldering is observed on both cover and foam core, the test is recorded as no ignition and the material passes the test.
WHAT IS PVC?
Polyvinyl Chloride (PVC) is one of the most used thermoplastic polymers worldwide. About 40 million tons of PVC are produced each year. PVC has been around longer than most plastics, first synthesized in 1872 and commercially produced by B.F. Goodrich Company in the 1920s. By comparison, many other common plastics were first synthesized and commercially viable only in the 1940s and 1950s. It is used most in the construction industry and is also used for signs, healthcare applications, and fibre for clothing.
PVC is a white, brittle solid. It can be made softer and more flexible by the addition of plasticizers.
Some of PVC plastic’s most important characteristics include:
- relatively low price (requires less energy to produce than most other types of industrial fabrics)
- resistance to environmental degradation
- water & chemical resistance (as well as to alkalis)
- high rigidity and hardness
- outstanding strength, durability & longevity (thanks to PVC’s molecular structure, which binds chlorine atoms to every other carbon chain. This is what makes PVC fabrics so resistant to oxidative reactions, enabling it to maintain performance over a long period of usage)
- ability to withstand abrasion and distortion
- flexibility
- versatility
- stable (PVC has an amorphous structure and contains halogens like chlorine and fluorine, that are known for being stable. This chemical stability is what enables flexible PVC to be so resistant to stressors like flame, chemicals, and oil)
PVC is manufactured in a range of weights and strengths. The Forté range of PVC fabrics ranges from 320gsm (grams per square meter) (PVC1) to over 1000gsm (PVC4). (an average t-shirt will rarely reach more than 200gsm)


Manufacturing
Forté Healthcare is the only Australian manufacturer producing specific medical grade covers, foam products and air systems within one single factory. This breadth of technology ensures a perfect alliance of technology for covers, foams and air systems.
Within the clinical setting, this ensures a better result for immersion, envelopment, microclimate, comfort and other factors necessary for skin integrity. This culmination of technology benefits other medical applications served by Forté including mental health, WH&S for Caregivers.
Forté proudly manufactures all elements of the high frequency welded covers within the Armidale factory. This ensures excellent quality control, efficiency, ability to customize and perfect compatibility with other components of the support surface.

You’re Covered by Forté Healthcare’s ‘Successful Solution Guarantee’
At Forté Healthcare, we are so confident in the care and performance our support surfaces deliver, that we back our products and expertise with our ‘Successful Solution Guarantee’.
Here’s how it works:
Our team of specialists will help you navigate our product range to correctly match our support surfaces with your care environment and patient risk profile. This ensures you achieve the highest levels of patient care and pressure injury prevention.
If, for whatever reason the product we recommend isn’t helping you achieve the outcome you expected, we’ll work with you until it is. And in the unlikely event we still cannot provide the right solution.
We’ll refund you 100% of the charges to date.