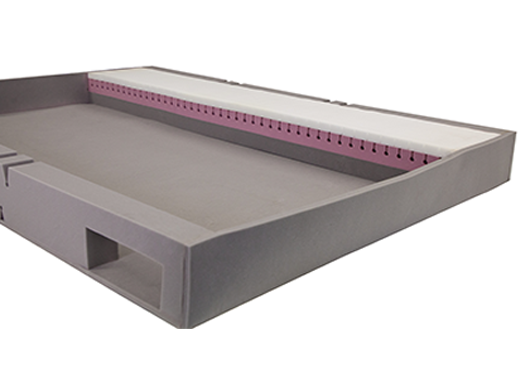
Bed Extension Bolsters
Finalé Medical Bed Bolsters
Finalé Medical Bed Bolsters are customisable and available in a wide range of options to meet with varied patient needs. Available in core structures from a single layer foam to multi-layered, high care configurations, Finalé Bolsters are manufactured from quality raw materials that meet and exceed clinical care requirements. Cover options include Heavy Duty PVC, Advantiflex Pressure Care Material and Mental Health Grade Material.
Features
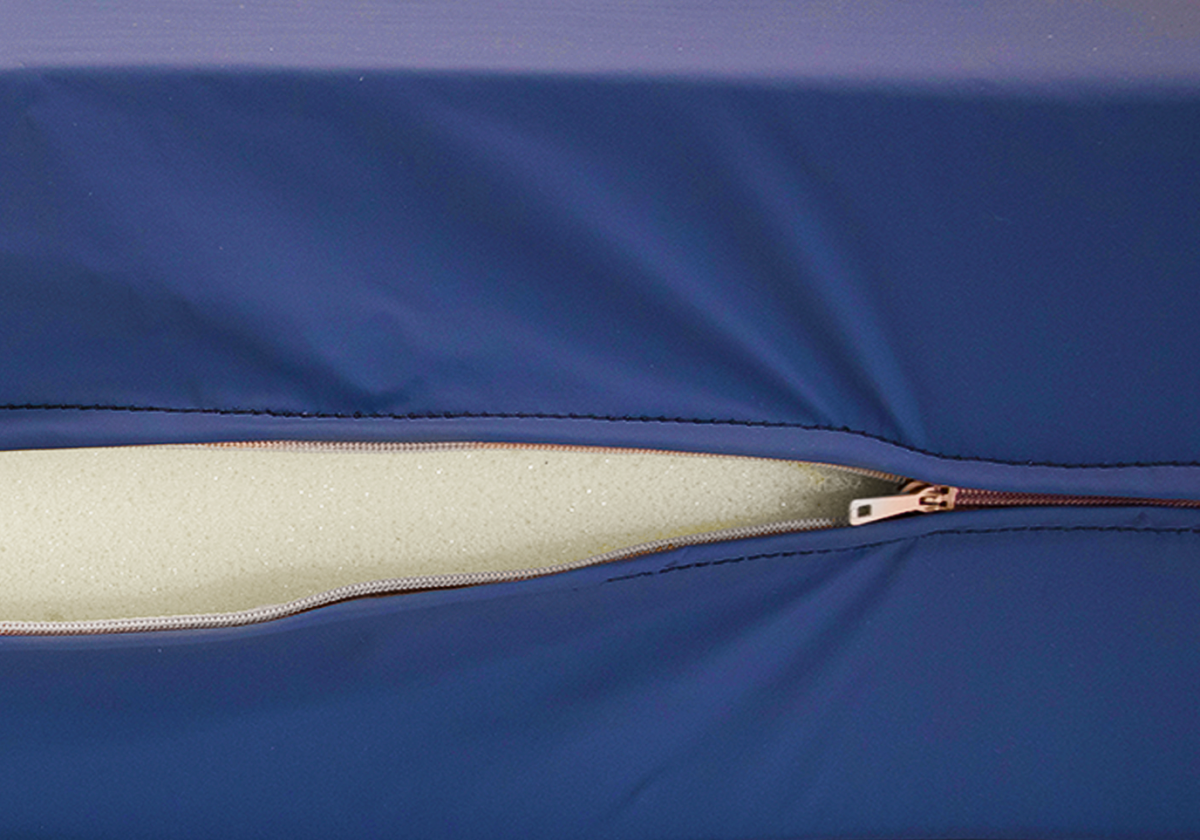
CONSTRUCTION
- Integrated robust zipper for cover removal
- Fluid penetration protection flap over zipper
- Robust seams

QUALITY FOAM CORE
- Supportive, durable
- Anti-microbial treatment
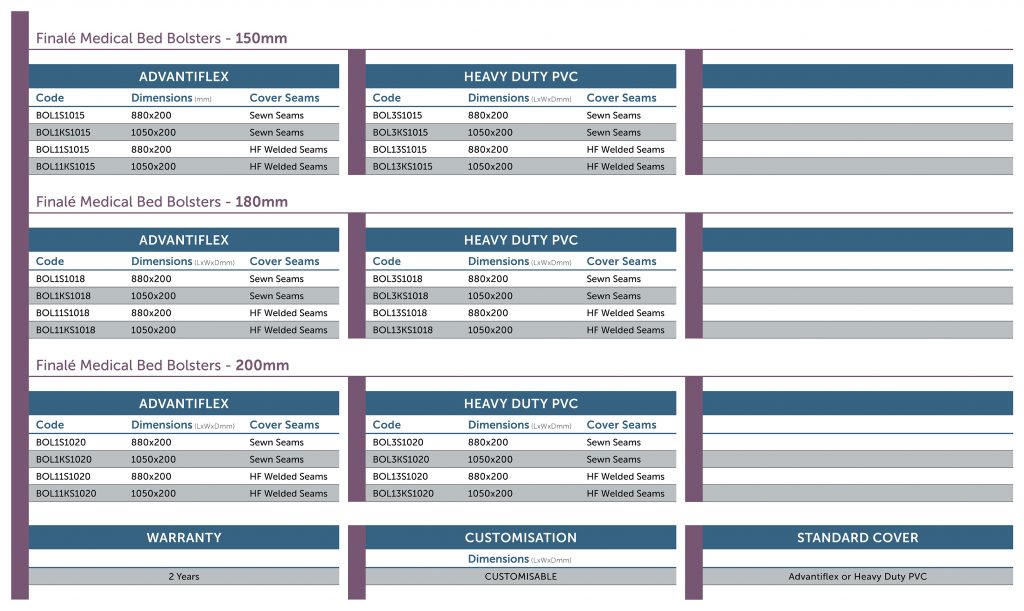
Specifications
Finalé Medical Bed Bolsters
Mattress & Bed Accessories

Soft Handles

Entrapment Protection
Hygienic

Safety
WHY USE A BED BOLSTER?
Bed extensions are used to extend a mattress platform or sleeping deck – both the width and length. Bed extensions ensure tall and wide people can remain comfortable, and over-sized beds can be moved safely throughout facilities
What mattresses do you use for beds with extensions in use? For long-term care, Forté can customise any mattress to fit the bed size perfectly. However, for short-term care, such as hospital usage, a custom size mattress is not a suitable and valuable solution. As soon as the patient has been discharged, the facility would be faced with either continuing to use the extra-large size bed, having to store away the large mattress, or discarding the mattress (which is a waste of money).
A solution for short-term care is bed bolsters – foam covered with a mattress cover in the exact shape of the bed extension to literally ‘fill the gap’. Finalé bolsters are customisable to suit any bed and extension.

IMPORTANCE OF PATIENT SAFETY
According to the World Health Organisation, Patient Safety is a health care discipline that emerged with the evolving complexity in health care systems and the resulting rise of patient harm in health care facilities. It aims to prevent and reduce risks, errors and harm that occur to patients during the provision of health care. A cornerstone of the discipline is a continuous improvement based on learning from errors and adverse events.
The occurrence of adverse events due to unsafe care is likely one of the 10 leading causes of death and disability in the world. In high-income countries, it is estimated that one in every 10 patients is harmed while receiving hospital care. The harm can be caused by a range of adverse events, with nearly 50% of them being preventable. Each year, 134 million adverse events occur in hospitals in low- and middle-income countries (LMICs), due to unsafe care, resulting in 2.6 million deaths.
Globally, as many as 4 in 10 patients are harmed in primary and outpatient health care. Up to 80% of harm is preventable. The most detrimental errors are related to diagnosis, prescription and the use of medicines.
Investments in reducing patient harm can lead to significant financial savings, and more importantly better patient outcomes. An example of prevention is engaging patients, if done well, it can reduce the burden of harm by up to 15%.

INFECTION CONTROL
Effective infection prevention and control are central to providing high quality healthcare for patients and a safe working environment for those who work in healthcare settings. The Australian Guidelines for the Prevention and Control of Infection in Healthcare (the guidelines) provide evidence-based recommendations that outline the critical aspects of infection prevention and control, focusing on core principles and priority areas for action.
The guidelines describe the best way to prevent and reduce infections occurring in healthcare settings including resistant infections. The guidelines include how to manage common infectious agents, for example, gastrointestinal viruses and evolving infectious agents, for example, influenza or multi-drug resistant organisms.
Any infectious agents introduced into the body can establish infection. In all healthcare settings, reusable medical devices, such as mattresses, should be handled in a manner that will prevent patients, healthcare workers and environmental contact with potentially infectious material. Post COVID-19 pandemic, Cleaning and infection control have become a bigger concern than ever before.
Appropriate reprocessing or precautions must be implemented for reusable equipment, such as the mattress to prevent patient-to-patient transmission of infectious agents.
Principles of reprocessing reusable medical devices include:
- Only Therapeutic Goods Administration (TGA)-included reusable medical devices should be used; before purchase, healthcare facilities should ensure that manufacturer’s reprocessing instructions are provided and are able to be followed by the healthcare facility.
- All reusable medical devices and patient-care equipment used in the clinical environment must be reprocessed according to their intended use and manufacturer’s advice.
However, Other standard precautions must take place. Standard precautions are basic infection prevention and control strategies that apply to everyone, regardless of their perceived or confirmed infectious status. Strategies include hand hygiene, personal protective equipment, cleaning, and appropriate handling and disposal of sharps. These are the first-line approach to infection prevention and control in health service organisations and are routinely applied as an essential strategy for minimising the spread of infections. Standard precautions minimise the risk of transmission of infectious agents from one person or place to another, even in high-risk situations, and render and maintain objects and areas as free as possible from infectious agents
Specifically, in relation to the support surface or any related accessories, there should be no exposed materials that will absorb fluid. As such, every mattress cover in the facility environment must have high frequency welded seams (as opposed to sewn) to meet infection control requirements and prevent seepage of fluid through open sewn seams. These electromagnetically fused seams protect against the risk of penetration fluid as they 100% seal off to prevent ingress of fluid or infection. To ensure extra strength, Forté reinforces welded seams with additional heavy duty stitching at stress points within the cover.
In addition, the Finalé bolster covers and foam cores are treated with anti-microbial treatment to prevent the growth of mould and bacteria on soft surfaces. It is a recommendation that soft surfaces must be impregnated with antimicrobial (self-disinfecting) materials.
All Forté products are included and registered on the Australian Register of Therapeutic Goods (ARTG). Unless exempt, reusable medical devices must be ‘included’ onto the ARTG before they may be supplied in Australia.
Forté mattresses can withstand stringent infection control procedures and have been designed to suit facility standards so you can have peace of mind that all areas of your facility are a safe, clean environments for staff and patients.

VERSATILITY BY BEING AUSTRALIAN MADE
Australian made by Forté Healthcare in Armidale NSW, the Finalé accessories are synonymous with quality, performance, longevity, and functionality and is trusted by leading Hospitals and healthcare professionals to deliver clinical results and positive outcomes.
Forté manufactures Finalé accessories to order which means they can be customised in size and configuration to suit a specific individual. Each patient has different anatomy which means one device is not always effective for all individuals.
Why is it important to purchase Australian made products?
By purchasing products from Forté you are investing in the future of pressure injury prevention in Australia, creating regional jobs and boosting our economy for the better of everyone. Australian Made for Australian Care.


Manufacturing
Leveraging clinical expertise, with leading raw material technology and in-house manufacturing capabilities Forté Healthcare ensures optimal outcomes for pressure injury prevention, infection control and patient comfort.

Hear what Health Professionals say About Forté Healthcare Mattresses…

You’re Covered by Forté Healthcare’s ‘Successful Solution Guarantee’
At Forté Healthcare, we are so confident in the care and performance our support surfaces deliver, that we back our products and expertise with our ‘Successful Solution Guarantee’.
Here’s how it works:
Our team of specialists will help you navigate our product range to correctly match our support surfaces with your care environment and patient risk profile. This ensures you achieve the highest levels of patient care and pressure injury prevention.
If, for whatever reason the product we recommend isn’t helping you achieve the outcome you expected, we’ll work with you until it is. And in the unlikely event we still cannot provide the right solution.
We’ll refund you 100% of the charges to date.